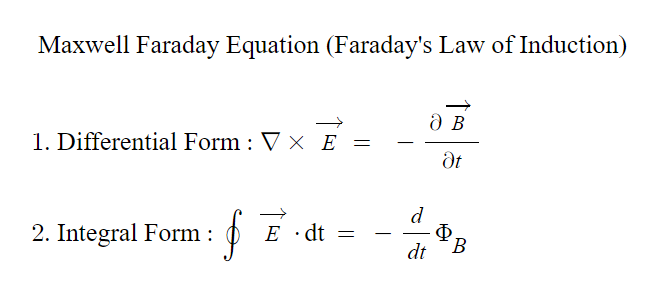History of Science - Michael Faraday

Life
- He was born on September 22, 1791, in Newington Butts, Surrey, England, and grew up in a poor family.
His family had to move to London due to French Revolution, and their situation worsened.
Faraday’s father worked as an apprentice to the village blacksmith, and his family initially lived in Gilber’s Row before moving to another village, jacob’s Well Mews, about five yaears later. - Faraday received only a basic education and began working as an apprentice bookbinder at an early age when he was 13.
On August 7, 1805, he entered into a seven-year apprenticeship contract with George Riebau.
- As was customary at the time, he lived with his master. Faraday was a skilled, open-minded, and curious apprentice. His curiosity made him read a lot in the bookstore. During his seven-year apprenticeship Faraday read many books, including Isaac Watt’s The Improvement of the Mind, and he enthusiastically implemented the principles and suggestions contained therein. In this book, Isaac recommended reading and taking notes on lectures, and then suggested sharing ideas with like-minded individuals.

- Faraday began attending evening lectures organized by the Philosophical Society in the City of London. These were public science lectures, and he subsequently engaged in discussions and study groups with fellow attendees he met there.
- In 1812, at 20, after finishing his apprenticeship, Faraday attended lectures by chemist Humphry Davy of the Royal Institution from the City Philosophical Society. Faraday sent Davy a 300-page book based on his lecture notes, earning Davy’s immediate and favorable response. Following an accident in 1813 that damaged Davy’s eyesight, he hired Faraday as an assistant
Achievement in Chemsitry
- As Humphry Davy’s assistant, Michael Faraday studied chlorine. He formed two new compounds with
chlorine and carbon, liquefied chlorine under high pressure, and successfully liquefied various
substances like ammonia, carbon dioxide, and sulfur dioxide
- Faraday discovered the concept of a critical point, revealing that above a certain temperature, pressure doesn’t alter a substance’s state
- He also discovered a chemical substance, benzene
Faraday devised an experiment by securing a wire through which currents flowed and moving a magnet around it. Finally, on September 3rd, he succeeded in the experiment, concluding that ‘the wire always moves perpendicularly to the magnet and distinctly revolves around it in a circular motion - Shortly after its publication, Woolaston and Davy expressed doubts about Faraday’s writing. They accused Faraday of plagiarizing Wollaston’s idea of ‘electromagnetic rotation’ without clear attribution. However, Faraday’s experimental demonstration was entirely different from Wollaston’s proposed method, and later, even Wollaston acknowledged this
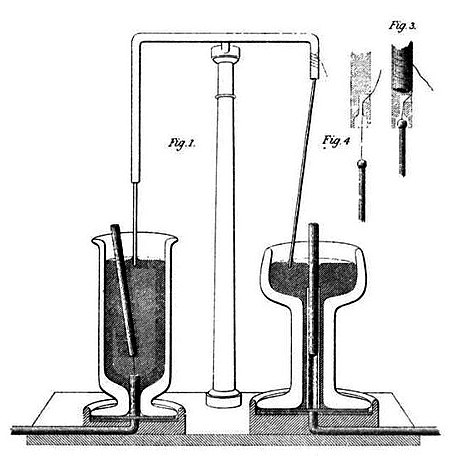
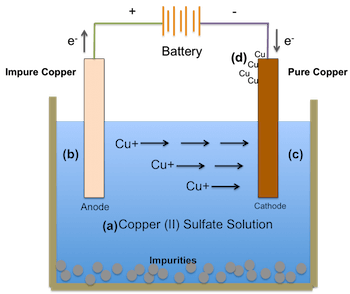
- Faraday’s First Law of electrolysis: He measured the volume of gases released when an electric current passed through a solution using a voltameter. Faraday passed the current through a cup containing diluted sulfuric acid and experimented with various conductors to induce currents into or out of the solution. The results confirmed his anticipation that the extent of electrochemical action depended solely on the quantity of current
- Faraday’s Second Law of electrolysis: Continuing into late 1833, Faraday commenced experiments in water with various substances dissolved in a voltmeter. He soon rediscovered the law that ‘the quantity of a substance decomposed by a fixed amount of electricity depends on the chemical equivalent of the precipitated or deposited substance. Having discovered these two laws on electrolysis, Faraday is credited with the unit of ‘faraday,’ representing the quantity of electricity needed to deposit one chemical equivalent. (Also known as Faradays’ constant, which is 1F = 96,485 C/mol)
Achievement in Physics
- Faraday’s cage: A Faraday cage is an enclosure made of conductive material, such as metal, that’s designed to block electromagnetic fields. This cage operates by redistributing electric charges and currents, thus preventing external electromagnetic radiation or signals from entering or exiting the enclosed space. The design of a Faraday cage ensures that any electromagnetic radiation that strikes the cage is distributed around the exterior, leaving the interior unaffected. It’s commonly used in various applications, such as shielding sensitive electronic equipment from electromagnetic interference, securing against lightning strikes.
- Polarisation: Faraday was curious about how light and electricity were related and studied the effects of an electric field on the polarization plane. When the polarization plane passed through a crystal, it rotated at a specific angle, a phenomenon easily observed. However, he couldn’t observe this effect in an electric field. Consequently, he aimed to convert the electric field into a magnetic field to investigate the influence of the magnetic field on the polarization plane.
- He suspended an optical glass in the magnetic field and passed the polarization through the glass. However, at that time, the magnetic field was too weak to observe a significant effect.
- On October 18th, Faraday defined a physical relationship between magnetism and light using a powerful electromagnet obtained from the Royal Military Academy in Woolwich. The phenomenon of the rotation of the polarization plane in a magnetic field, discovered by Faraday, was named the Faraday Effect
3. Electromagnetic induction: One of his most influential discoveries was demonstrating how a changing magnetic field could induce an electric current. He observed that by moving a magnet in and out of a coil of wire, it produced an electric current in the wire. This laid the groundwork for generating electricity through generators and transformers.
More Stories
- Faraday was an exceptional experimental physicist who could articulate his ideas in simple and clear language. However, his mathematical abilities only extended to basic algebra, and he did not handle trigonometry. James Clerk Maxwell consolidated the contributions of scientists, including Faraday’s, into a set of fundamental equations known as Maxwell’s equations, which form the basis of modern electromagnetism